Tutorial at first release (v1.0.1)#
Basic settings#
[1]:
import numpy as np
import pandas as pd
import scanpy as sc
[2]:
sc.settings.verbosity = 3
sc.logging.print_header()
sc.settings.set_figure_params(dpi=80, facecolor='white')
scanpy==1.6.1 anndata==0.7.5 umap==0.4.6 numpy==1.19.5 scipy==1.5.3 pandas==1.1.5 scikit-learn==0.24.0 statsmodels==0.12.1 python-igraph==0.8.3 louvain==0.7.0 leidenalg==0.8.3
[35]:
save_dir='./' ## need to change the directory
sc.settings.figdir = save_dir
Import data#
The data could be downloaded from https://drive.google.com/drive/folders/1MZEaM28PHW4FTSKzy-h_OqPnSlRKiYKW?usp=sharing.
[3]:
%%time
adata=sc.read('./Hochgerner_dentate_gyrus_QC.h5ad') ## need to change the directory
CPU times: user 174 ms, sys: 237 ms, total: 412 ms
Wall time: 410 ms
[4]:
np.unique(adata.obs['CellTypes'])
[4]:
array(['Astro-adult', 'Astro-juv', 'CA3-Pyr', 'Cajal-Retzius',
'Endothelial', 'Ependymal', 'GABA', 'GC-adult', 'GC-juv',
'Immature-Astro', 'Immature-GABA', 'Immature-GC', 'Immature-Pyr',
'MOL', 'MiCajal-Retziusoglia', 'NFOL', 'Neuroblast', 'OPC', 'PVM',
'RGL', 'RGL_young', 'VLMC', 'nIPC', 'nIPC-perin'], dtype=object)
[5]:
len(np.unique(adata.obs['CellTypes']))
[5]:
24
[6]:
adata
[6]:
AnnData object with n_obs × n_vars = 23025 × 19444
obs: 'source name', 'organism', 'characteristics: strain', 'characteristics: age', 'characteristics: sex of pooled animals', 'characteristics: cell cluster', 'molecule', 'SRR run accession', 'raw file (original file name)', 'UMI_CellularBarcode', 'CellTypes', 'n_genes', 'n_genes_by_counts', 'total_counts', 'total_counts_mt', 'pct_counts_mt'
var: 'n_cells', 'mt', 'n_cells_by_counts', 'mean_counts', 'pct_dropout_by_counts', 'total_counts'
uns: 'CellTypes_colors'
Note: the data is already filtered after proper quality control.
Normalization#
[7]:
sc.pp.normalize_total(adata, target_sum=1e4)
sc.pp.log1p(adata)
normalizing counts per cell
finished (0:00:00)
HVG identification#
[8]:
sc.pp.highly_variable_genes(adata,
n_top_genes=3000
)
print(sum(adata.var.highly_variable))
sc.pl.highly_variable_genes(adata)
If you pass `n_top_genes`, all cutoffs are ignored.
extracting highly variable genes
finished (0:00:01)
--> added
'highly_variable', boolean vector (adata.var)
'means', float vector (adata.var)
'dispersions', float vector (adata.var)
'dispersions_norm', float vector (adata.var)
3000
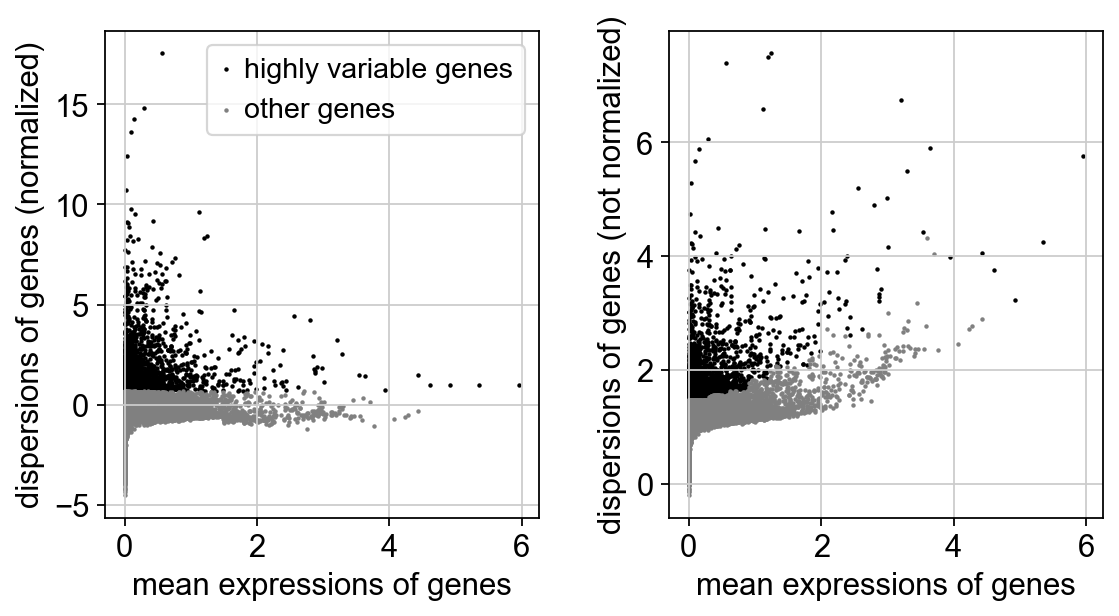
Run PCA#
[9]:
use_highly_variable=True
from sklearn.preprocessing import StandardScaler
expr = adata[:, adata.var['highly_variable']].X if use_highly_variable else adata.X
expr=StandardScaler(with_mean=False).fit_transform(expr)
expr[expr > 10] = 10
from sklearn.decomposition import TruncatedSVD
transformer = TruncatedSVD(n_components=50, random_state=42)
adata.obsm['X_pca']= transformer.fit_transform(expr)
[10]:
sc.pl.pca(adata,color='CellTypes')
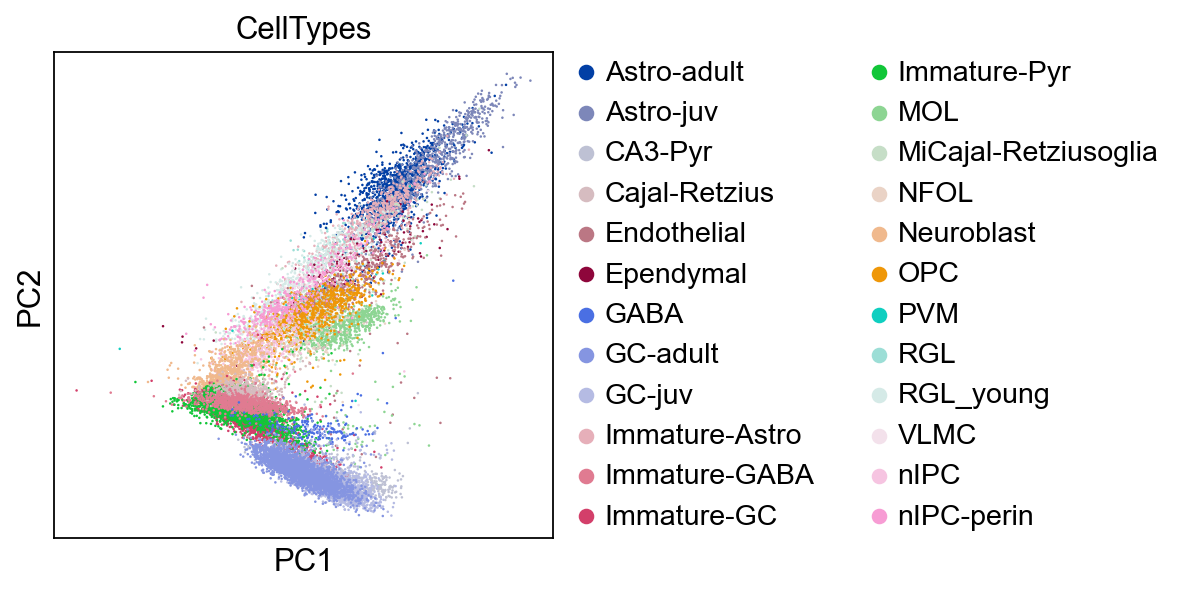
Reorder the CellTypes#
[11]:
%%time
sc.tl.dendrogram(adata,groupby='CellTypes',use_rep='X_pca')
Storing dendrogram info using `.uns["dendrogram_['CellTypes']"]`
CPU times: user 11.5 ms, sys: 3.96 ms, total: 15.4 ms
Wall time: 13.6 ms
[12]:
celltype=adata.obs['CellTypes'].values.copy()
new_order=adata.uns["dendrogram_['CellTypes']"]['categories_ordered']
celltype=celltype.reorder_categories(new_order)
[13]:
adata.obs['CellTypes']=celltype
Run UMAP#
[14]:
%%time
sc.pp.neighbors(adata,
n_neighbors=15,random_state=10,knn=True,
method="umap")
computing neighbors
using 'X_pca' with n_pcs = 50
finished: added to `.uns['neighbors']`
`.obsp['distances']`, distances for each pair of neighbors
`.obsp['connectivities']`, weighted adjacency matrix (0:00:20)
CPU times: user 33.4 s, sys: 16 s, total: 49.4 s
Wall time: 20.9 s
[15]:
%%time
sc.tl.umap(adata)
computing UMAP
finished: added
'X_umap', UMAP coordinates (adata.obsm) (0:00:14)
CPU times: user 57.5 s, sys: 2min 26s, total: 3min 23s
Wall time: 14.7 s
[16]:
%%time
sc.pl.umap(adata,
color=['CellTypes'],
palette=sc.pl.palettes.default_102,
size=10,
frameon=True)
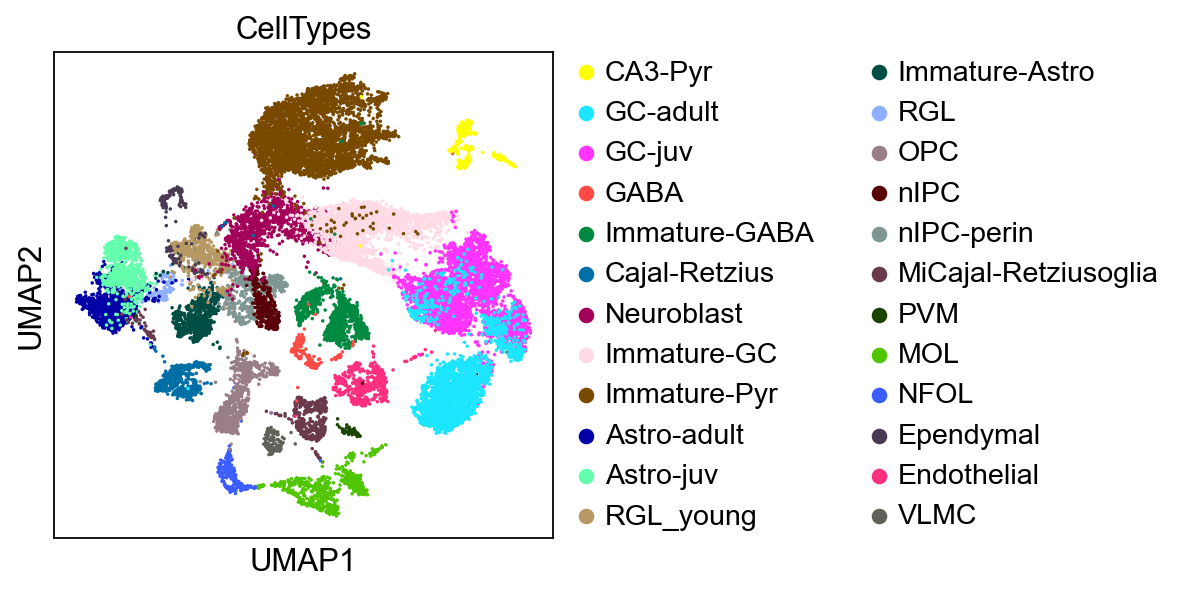
CPU times: user 1.34 s, sys: 48.9 ms, total: 1.38 s
Wall time: 1.38 s
Marker gene identification#
1. COSG#
Import COSG:
[46]:
import cosg as cosg
import importlib
importlib.reload(cosg)
[46]:
<module 'cosg' from '/platinum-2/daimin/analysis/jupyter/python/cosg.py'>
Print the usgae:
[47]:
help(cosg.cosg)
Help on function cosg in module cosg:
cosg(adata, groupby='CellTypes', groups:Union[typing_extensions.Literal['all'], Iterable[str]]='all', mu=1, remove_lowly_expressed:bool=False, expressed_pct:Union[float, NoneType]=0.1, n_genes_user:int=50, key_added:Union[str, NoneType]=None, use_raw:bool=True, layer:Union[str, NoneType]=None, reference:str='rest', copy:bool=False)
Marker gene identification for single-cell sequencing data using COSG.
Parameters
----------
adata
Annotated data matrix. Note: input paramters are simliar to the parameters used for scanpy's rank_genes_groups() function.
groupby
The key of the cell groups in .obs, the default value is set to 'CellTypes'.
groups
Subset of cell groups, e.g. [`'g1'`, `'g2'`, `'g3'`], to which comparison shall be restricted. The default value is 'all', and all groups will be compared.
mu
The penalty restricting marker genes expressing in non-target cell groups. Larger value represents more strict restrictions. mu should be >= 0, and by default, mu = 1.
remove_lowly_expressed
If True, genes that express a percentage of target cells smaller than a specific value (`expressed_pct`) are not considered as marker genes for the target cells. The default value is False.
expressed_pct
When `remove_lowly_expressed` is set to True, genes that express a percentage of target cells smaller than a specific value (`expressed_pct`) are not considered as marker genes for the target cells. The default value for `expressed_pct`
is 0.1 (10%).
n_genes_user
The number of genes that appear in the returned tables. The default value is 50.
key_added
The key in `adata.uns` information is saved to.
use_raw
Use `raw` attribute of `adata` if present.
layer
Key from `adata.layers` whose value will be used to perform tests on.
reference
If `'rest'`, compare each group to the union of the rest of the group.
If a group identifier, compare with respect to this group.
Returns
-------
**names** : structured `np.ndarray` (`.uns['rank_genes_groups']`)
Structured array to be indexed by group id storing the gene names. Ordered according to scores.
**scores** : structured `np.ndarray` (`.uns['rank_genes_groups']`)
Structured array to be indexed by group id storing COSG scores for each gene for each
group. Ordered according to scores.
Notes
-----
Contact: daimin@zju.edu.cn
Examples
--------
>>> import cosg as cosg
>>> import scanpy as sc
>>> adata = sc.datasets.pbmc68k_reduced()
>>> cosg.cosg(adata, key_added='cosg', groupby='bulk_labels')
>>> sc.pl.rank_genes_groups(adata, key='cosg')
[49]:
%%time
import time
t0= time.clock()
cosg.cosg(adata,
key_added='cosg',
mu=1,
n_genes_user=50,
groupby='CellTypes')
runtime_cosg = time.clock() - t0
**finished identifying marker genes by COSG**
CPU times: user 2.7 s, sys: 343 ms, total: 3.04 s
Wall time: 3.04 s
[50]:
sc.tl.dendrogram(adata,groupby='CellTypes',use_rep='X_pca')
Storing dendrogram info using `.uns["dendrogram_['CellTypes']"]`
[51]:
sc.pl.rank_genes_groups_dotplot(adata,groupby='CellTypes',
cmap='Spectral_r',
standard_scale='var',
n_genes=3,key='cosg')
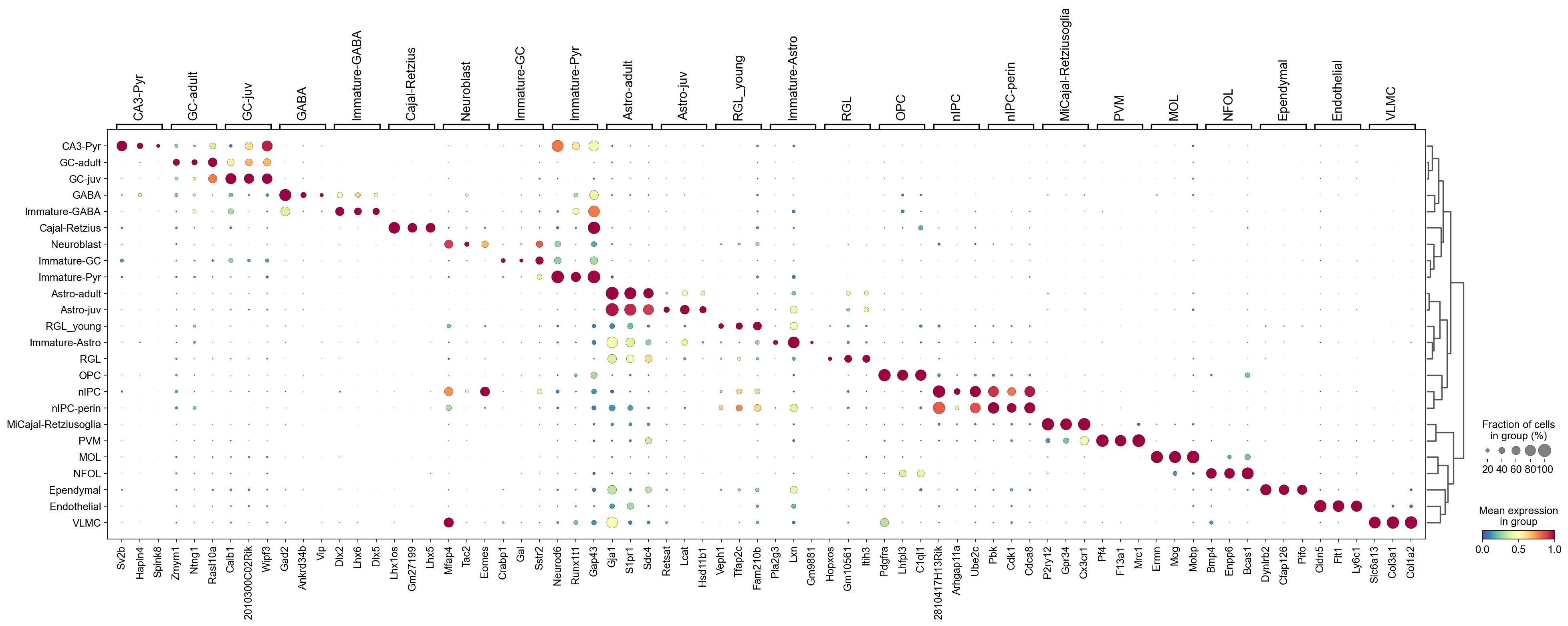
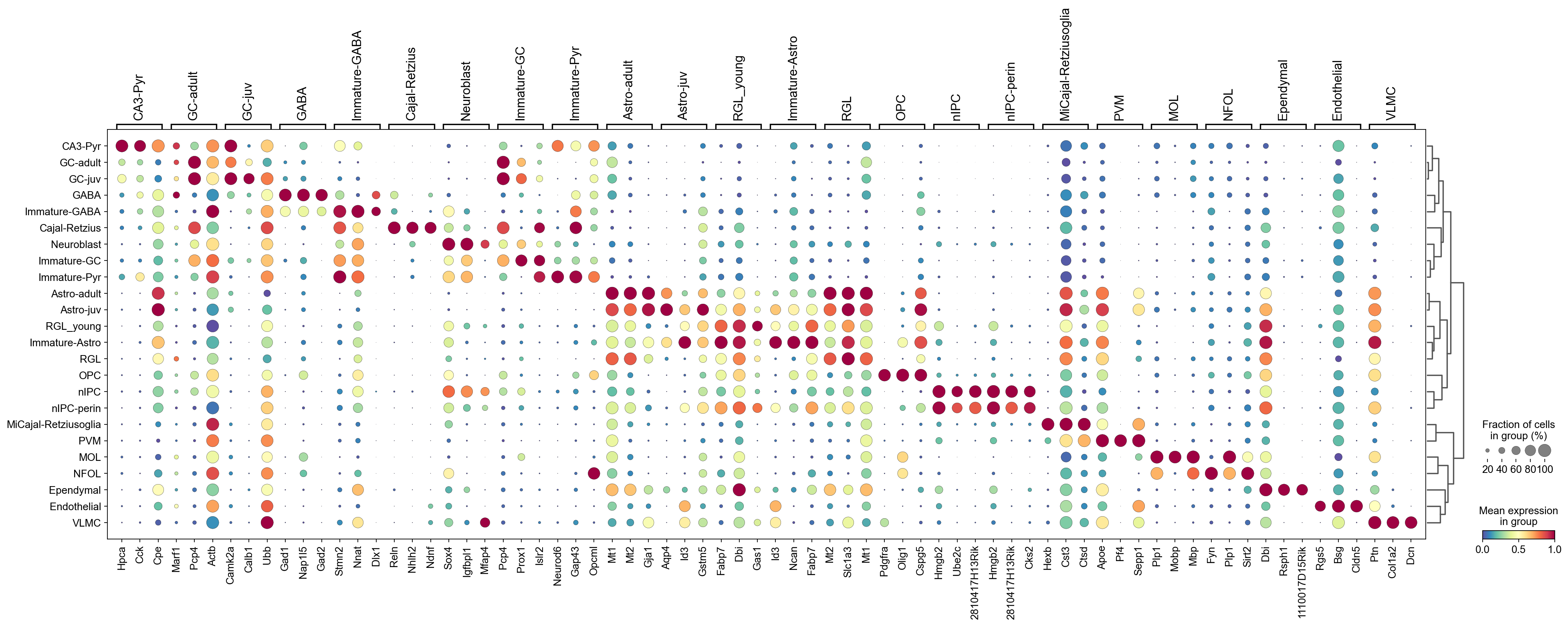
2. Logistic regression#
[29]:
%%time
t0= time.clock()
sc.tl.rank_genes_groups(adata,
groupby='CellTypes',
method='logreg',
key_added='logreg',
n_genes=50)
runtime_logreg = time.clock() - t0
ranking genes
finished: added to `.uns['logreg']`
'names', sorted np.recarray to be indexed by group ids
'scores', sorted np.recarray to be indexed by group ids
(0:03:16)
CPU times: user 15min 34s, sys: 52min 18s, total: 1h 7min 52s
Wall time: 3min 16s
/platinum-2/daimin/software/miniconda3/envs/scda3/lib/python3.6/site-packages/sklearn/linear_model/_logistic.py:765: ConvergenceWarning: lbfgs failed to converge (status=1):
STOP: TOTAL NO. of ITERATIONS REACHED LIMIT.
Increase the number of iterations (max_iter) or scale the data as shown in:
https://scikit-learn.org/stable/modules/preprocessing.html
Please also refer to the documentation for alternative solver options:
https://scikit-learn.org/stable/modules/linear_model.html#logistic-regression
extra_warning_msg=_LOGISTIC_SOLVER_CONVERGENCE_MSG)
[30]:
sc.pl.rank_genes_groups_dotplot(adata,groupby='CellTypes',
cmap='Spectral_r',
standard_scale='var',
n_genes=3,key='logreg')
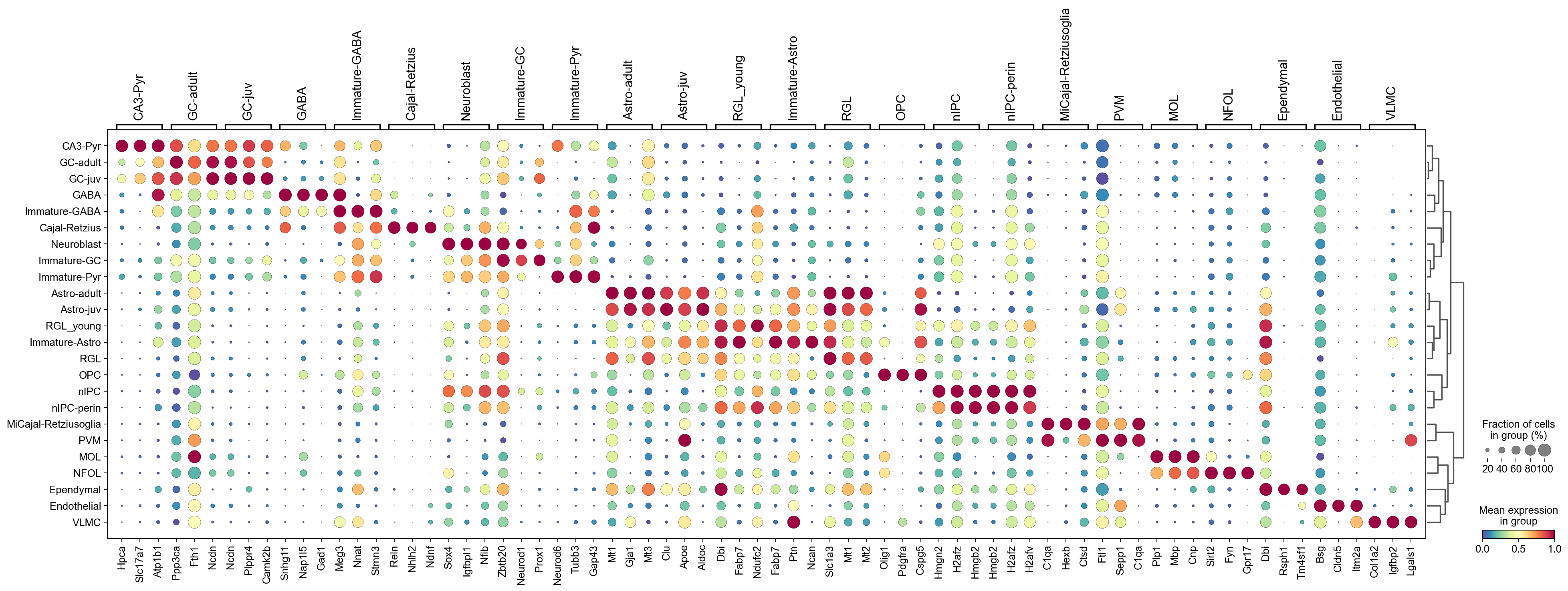
3. Wilcoxon-test#
[31]:
%%time
t0= time.clock()
sc.tl.rank_genes_groups(adata, groupby='CellTypes',
tie_correct=False,
method='wilcoxon',
key_added='wilcoxon',n_genes=50)
runtime_wilcoxon= time.clock() - t0
ranking genes
finished: added to `.uns['wilcoxon']`
'names', sorted np.recarray to be indexed by group ids
'scores', sorted np.recarray to be indexed by group ids
'logfoldchanges', sorted np.recarray to be indexed by group ids
'pvals', sorted np.recarray to be indexed by group ids
'pvals_adj', sorted np.recarray to be indexed by group ids (0:00:46)
CPU times: user 37.5 s, sys: 8.72 s, total: 46.2 s
Wall time: 46.2 s
[32]:
sc.pl.rank_genes_groups_dotplot(adata,groupby='CellTypes',
cmap='Spectral_r',
standard_scale='var',
n_genes=3,key='wilcoxon')
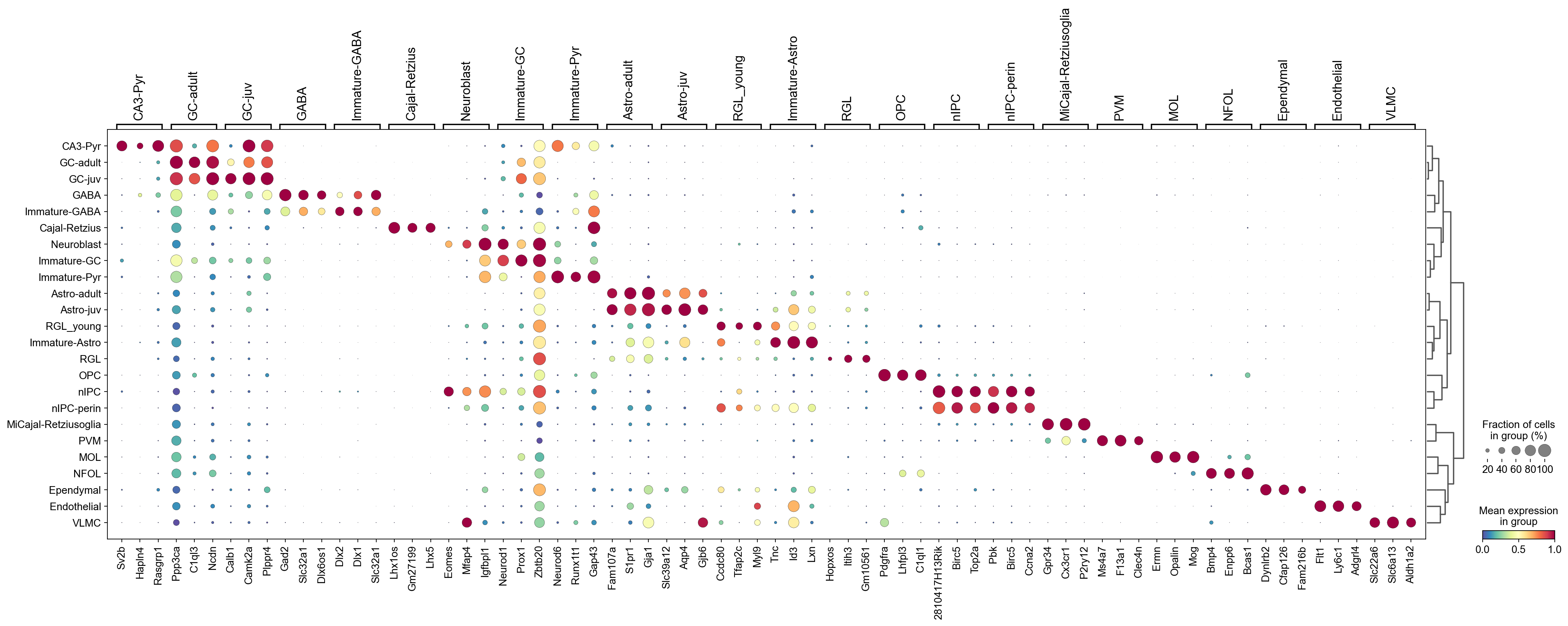
4. Wilcoxon-test (TIE)#
[33]:
%%time
t0= time.clock()
sc.tl.rank_genes_groups(adata, groupby='CellTypes',
tie_correct=True,
method='wilcoxon',
key_added='wilcoxon_tie',n_genes=50)
runtime_wilcoxon_tie = time.clock() - t0
ranking genes
/platinum-2/daimin/software/miniconda3/envs/scda3/lib/python3.6/site-packages/scanpy/tools/_rank_genes_groups.py:313: RuntimeWarning: invalid value encountered in true_divide
) / std_dev
finished: added to `.uns['wilcoxon_tie']`
'names', sorted np.recarray to be indexed by group ids
'scores', sorted np.recarray to be indexed by group ids
'logfoldchanges', sorted np.recarray to be indexed by group ids
'pvals', sorted np.recarray to be indexed by group ids
'pvals_adj', sorted np.recarray to be indexed by group ids (0:15:23)
CPU times: user 12min 45s, sys: 2min 38s, total: 15min 23s
Wall time: 15min 23s
[34]:
sc.pl.rank_genes_groups_dotplot(adata,groupby='CellTypes',
cmap='Spectral_r',
standard_scale='var',
n_genes=3,key='wilcoxon_tie')
Runtime comparison#
[71]:
print( [runtime_logreg, runtime_wilcoxon, runtime_wilcoxon_tie, runtime_cosg] )
[4072.48, 46.220000000000255, 923.6999999999998, 3.0299999999997453]
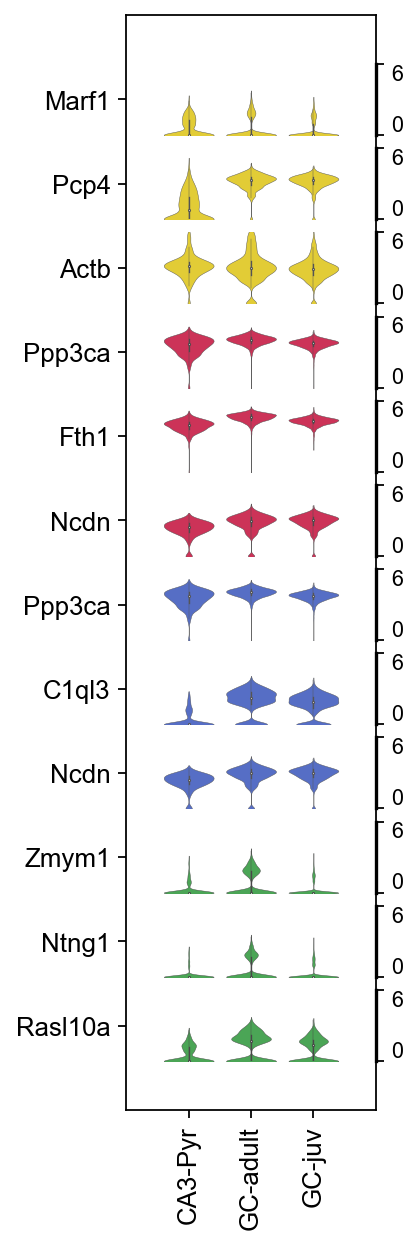
Violin plots#
[52]:
celltype_selected= 'GC-adult'
[53]:
adata_selected=adata[adata.obs['CellTypes'].isin(['CA3-Pyr', 'GC-adult', 'GC-juv'])]
[56]:
marker_violin=np.hstack([pd.DataFrame(adata.uns['logreg']['names'])[celltype_selected][:3].values,
pd.DataFrame(adata.uns['wilcoxon']['names'])[celltype_selected][:3].values,
pd.DataFrame(adata.uns['wilcoxon_tie']['names'])[celltype_selected][:3].values,
pd.DataFrame(adata.uns['cosg']['names'])[celltype_selected][:3].values])
[57]:
marker_violin
[57]:
array(['Marf1', 'Pcp4', 'Actb', 'Ppp3ca', 'Fth1', 'Ncdn', 'Ppp3ca',
'C1ql3', 'Ncdn', 'Zmym1', 'Ntng1', 'Rasl10a'], dtype=object)
[66]:
vp=sc.pl.stacked_violin(adata_selected,
marker_violin,
groupby='CellTypes',
swap_axes=True,
figsize=(4, 9),
scale='width',
yticklabels=True,
size=0.2,
inner='box',
cut=True,
return_fig=True,
)
[67]:
icolor=[ "#ffe119", # Logistic regression
"#e6194b", # Wilcoxon-test
"#4363d8", # Wilcoxon-test (TIE)
"#3cb44b" # COSG ,
]
icolor=np.hstack([np.repeat(i,3) for i in icolor])
icolor
[67]:
array(['#ffe119', '#ffe119', '#ffe119', '#e6194b', '#e6194b', '#e6194b',
'#4363d8', '#4363d8', '#4363d8', '#3cb44b', '#3cb44b', '#3cb44b'],
dtype='<U7')
[68]:
icolor=list(icolor)
[69]:
vp.style(ylim=(0,6),
row_palette=icolor,yticklabels=True,).show()
Save the data#
[36]:
adata.write(save_dir+'/COSG_tutorial_Hochgerner_dentate_gyrus_QC.h5ad')
