Pre-processing CellRanger outputs (multiple samples)#
This notebook demonstrates the pipeline for processing scRNA-seq data, covering metric computation, normalization, clustering, cell type prediction, and the identification/removal of doublets and low-quality clusters. The final output is pre-processed, annotated scRNA-seq data.
[1]:
import piaso
import cosg
/home/vas744/.local/lib/python3.9/site-packages/networkx/utils/backends.py:135: RuntimeWarning: networkx backend defined more than once: nx-loopback
backends.update(_get_backends("networkx.backends"))
[2]:
import os
import numpy as np
import pandas as pd
import scanpy as sc
import anndata as ad
import logging
from matplotlib import rcParams
import warnings
# To modify the default figure size, use rcParams.
rcParams['figure.figsize'] = 4, 4
rcParams['font.sans-serif'] = "Arial"
rcParams['font.family'] = "Arial"
sc.settings.verbosity = 3
sc.logging.print_header()
sc.set_figure_params(dpi=80,dpi_save=300, color_map='viridis',facecolor='white')
scanpy==1.10.3 anndata==0.10.8 umap==0.5.7 numpy==1.26.4 scipy==1.13.0 pandas==2.2.3 scikit-learn==1.5.2 statsmodels==0.14.4 igraph==0.11.5 louvain==0.8.2 pynndescent==0.5.13
[3]:
warnings.simplefilter(action='ignore', category=FutureWarning)
Load the data#
This dataset was obtained from a mouse at E18 and includes various samples derived from different sequencing technologies, tissue types, and cell vs. nuclei preparations.
[4]:
!/home/vas744/Software/gdrive files download --overwrite --recursive --destination /n/scratch/users/v/vas744/Data/Public/PIASO/multiple_samples 1fOVhYBDQirFtrpRPuy7euAHykXuTSe_r
Found 3 files in 1 directories with a total size of 129.2 MB
Creating directory E18_h5_files
Downloaded 3 files in 1 directories with a total size of 129.2 MB
For this tutorial, we will use only three samples from this dataset. The selection includes one sample from cells, one from nuclei, and one from a different version of 10x (GEM-X-v4), providing a diverse range of sample types within a limited dataset.
[4]:
data_path='/n/scratch/users/v/vas744/Data/Public/PIASO/multiple_samples/'
time_points = [entry.name for entry in os.scandir(data_path) if entry.is_dir()]
print(time_points)
for time_point in time_points:
samples = [entry.name for entry in os.scandir(data_path+time_point) if entry.is_dir()]
print(time_point, samples)
['E18']
E18 ['E18_v3.1_cell', 'E18_v3.1_nuclei', 'E18_v4_cell']
We will use this function to load multiple .h5 files into adata objects, creating a list of adata objects.
[5]:
def load_multiple_anndata(
data_path,
time_points,
key_added):
anndata_list = []
for time_point in time_points:
samples = [entry.name for entry in os.scandir(data_path+time_point) if entry.is_dir()]
for sample in samples:
try:
# Load the data
file_name = os.listdir(data_path+time_point+'/'+sample)[0]
h5_path=data_path+time_point+'/'+sample+'/'+file_name
if os.path.exists(h5_path):
adata = sc.read_10x_h5(h5_path)
print('Loading the h5 file')
else:
adata = sc.read_10x_mtx(data_path+'/'+sample, cache=True)
adata.var_names_make_unique()
adata.obs_names_make_unique()
adata.obs[key_added]=sample
anndata_list.append(adata)
print('Sample loaded: ', sample)
except Exception as e:
print(f"Error loading {data_path}: {e}")
return anndata_list
[6]:
adata_list=load_multiple_anndata(data_path=data_path, time_points=time_points, key_added='Sample')
reading /n/scratch/users/v/vas744/Data/Public/PIASO/multiple_samples/E18/E18_v3.1_cell/neuron_10k_v3_filtered_feature_bc_matrix.h5
(0:00:02)
/home/vas744/.local/lib/python3.9/site-packages/anndata/_core/anndata.py:1820: UserWarning: Variable names are not unique. To make them unique, call `.var_names_make_unique`.
utils.warn_names_duplicates("var")
Loading the h5 file
Sample loaded: E18_v3.1_cell
reading /n/scratch/users/v/vas744/Data/Public/PIASO/multiple_samples/E18/E18_v3.1_nuclei/SC3_v3_NextGem_DI_Nuclei_5K_SC3_v3_NextGem_DI_Nuclei_5K_count_sample_feature_bc_matrix.h5
/home/vas744/.local/lib/python3.9/site-packages/anndata/_core/anndata.py:1820: UserWarning: Variable names are not unique. To make them unique, call `.var_names_make_unique`.
utils.warn_names_duplicates("var")
(0:00:00)
/home/vas744/.local/lib/python3.9/site-packages/anndata/_core/anndata.py:1820: UserWarning: Variable names are not unique. To make them unique, call `.var_names_make_unique`.
utils.warn_names_duplicates("var")
Loading the h5 file
Sample loaded: E18_v3.1_nuclei
reading /n/scratch/users/v/vas744/Data/Public/PIASO/multiple_samples/E18/E18_v4_cell/10k_Mouse_Neurons_3p_gemx_10k_Mouse_Neurons_3p_gemx_count_sample_filtered_feature_bc_matrix.h5
/home/vas744/.local/lib/python3.9/site-packages/anndata/_core/anndata.py:1820: UserWarning: Variable names are not unique. To make them unique, call `.var_names_make_unique`.
utils.warn_names_duplicates("var")
(0:00:02)
/home/vas744/.local/lib/python3.9/site-packages/anndata/_core/anndata.py:1820: UserWarning: Variable names are not unique. To make them unique, call `.var_names_make_unique`.
utils.warn_names_duplicates("var")
Loading the h5 file
Sample loaded: E18_v4_cell
/home/vas744/.local/lib/python3.9/site-packages/anndata/_core/anndata.py:1820: UserWarning: Variable names are not unique. To make them unique, call `.var_names_make_unique`.
utils.warn_names_duplicates("var")
Concatenate the adata objects in the list to create a single adata object that contains information from all the samples and time points.
[7]:
adata=ad.concat(adata_list, join='outer',index_unique="-")
[8]:
adata
[8]:
AnnData object with n_obs × n_vars = 30257 × 37089
obs: 'Sample'
[9]:
adata.layers['raw']=adata.X.copy()
[10]:
adata.var_names_make_unique()
Next, we filter out cells with fewer than 200 detected genes.
[11]:
sc.pp.filter_cells(adata, min_genes=200)
filtered out 386 cells that have less than 200 genes expressed
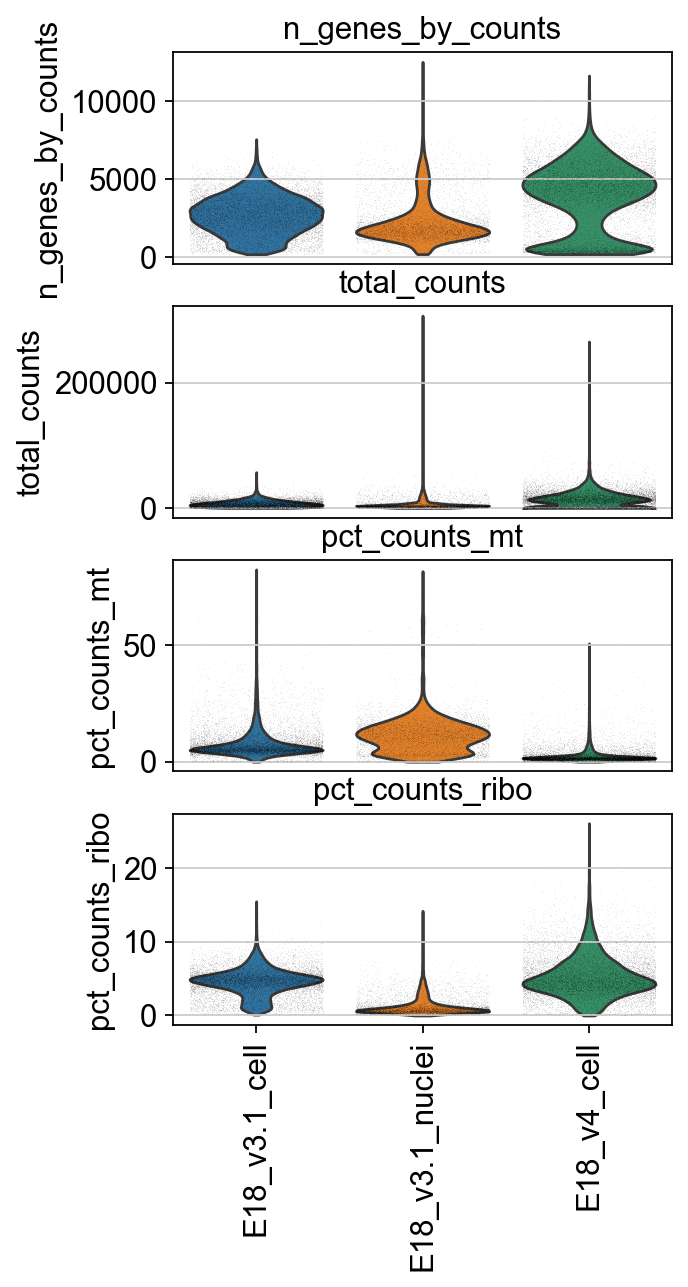
We identify mitochondrial and ribosomal protein genes and compute their proportion in each cell’s total read count. A high proportion of these reads often indicates low-quality cells.
[12]:
adata.var['mt'] = adata.var_names.str.startswith('mt-') # annotate the group of mitochondrial genes as 'mt'
sc.pp.calculate_qc_metrics(adata, qc_vars=['mt'], percent_top=None, log1p=False, inplace=True)
[13]:
ribo_cells = adata.var_names.str.startswith('Rps','Rpl')
adata.obs['pct_counts_ribo'] = np.ravel(100*np.sum(adata[:, ribo_cells].X, axis = 1) / np.sum(adata.X, axis = 1))
[14]:
piaso.pl.plot_features_violin(adata,
['n_genes_by_counts', 'total_counts', 'pct_counts_mt','pct_counts_ribo'],
width_single=4,
groupby='Sample')
Doublet prediction#
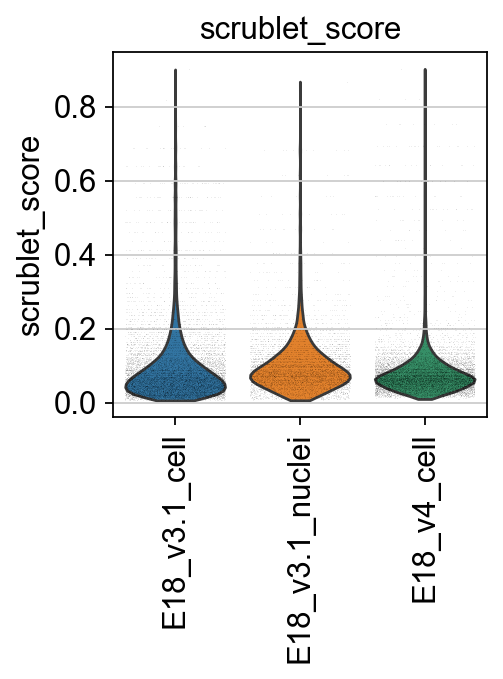
Next, we compute the Scrublet score to identify and predict potential doublets.
[15]:
experiments=np.unique(adata.obs['Sample'])
adata.obs['scrublet_score']=np.repeat(0,adata.n_obs)
adata.obs['predicted_doublets']=np.repeat(False,adata.n_obs)
[16]:
import scrublet as scr
for experiment in experiments:
print(experiment)
adatai=adata[adata.obs['Sample']==experiment]
scrub = scr.Scrublet(adatai.X.todense(),random_state=10)
doublet_scores, predicted_doublets = scrub.scrub_doublets()
adata.obs['predicted_doublets'][adatai.obs_names]=predicted_doublets
adata.obs['scrublet_score'][adatai.obs_names]=doublet_scores
E18_v3.1_cell
Preprocessing...
Simulating doublets...
Embedding transcriptomes using PCA...
Calculating doublet scores...
Automatically set threshold at doublet score = 0.39
Detected doublet rate = 2.3%
Estimated detectable doublet fraction = 28.0%
Overall doublet rate:
Expected = 10.0%
Estimated = 8.1%
Elapsed time: 32.3 seconds
E18_v3.1_nuclei
/tmp/ipykernel_17593/2312138584.py:10: SettingWithCopyWarning:
A value is trying to be set on a copy of a slice from a DataFrame
See the caveats in the documentation: https://pandas.pydata.org/pandas-docs/stable/user_guide/indexing.html#returning-a-view-versus-a-copy
adata.obs['predicted_doublets'][adatai.obs_names]=predicted_doublets
/tmp/ipykernel_17593/2312138584.py:12: SettingWithCopyWarning:
A value is trying to be set on a copy of a slice from a DataFrame
See the caveats in the documentation: https://pandas.pydata.org/pandas-docs/stable/user_guide/indexing.html#returning-a-view-versus-a-copy
adata.obs['scrublet_score'][adatai.obs_names]=doublet_scores
Preprocessing...
Simulating doublets...
Embedding transcriptomes using PCA...
Calculating doublet scores...
Automatically set threshold at doublet score = 0.74
Detected doublet rate = 0.0%
Estimated detectable doublet fraction = 0.5%
Overall doublet rate:
Expected = 10.0%
Estimated = 3.3%
Elapsed time: 17.2 seconds
E18_v4_cell
/tmp/ipykernel_17593/2312138584.py:10: SettingWithCopyWarning:
A value is trying to be set on a copy of a slice from a DataFrame
See the caveats in the documentation: https://pandas.pydata.org/pandas-docs/stable/user_guide/indexing.html#returning-a-view-versus-a-copy
adata.obs['predicted_doublets'][adatai.obs_names]=predicted_doublets
Preprocessing...
Simulating doublets...
Embedding transcriptomes using PCA...
Calculating doublet scores...
Automatically set threshold at doublet score = 0.46
Detected doublet rate = 0.9%
Estimated detectable doublet fraction = 15.3%
Overall doublet rate:
Expected = 10.0%
Estimated = 5.7%
Elapsed time: 65.7 seconds
/tmp/ipykernel_17593/2312138584.py:10: SettingWithCopyWarning:
A value is trying to be set on a copy of a slice from a DataFrame
See the caveats in the documentation: https://pandas.pydata.org/pandas-docs/stable/user_guide/indexing.html#returning-a-view-versus-a-copy
adata.obs['predicted_doublets'][adatai.obs_names]=predicted_doublets
[17]:
piaso.pl.plot_features_violin(adata,
['scrublet_score'],
groupby='Sample',
width_single=3,
height_single=3)
[18]:
tmp=np.repeat(False, adata.n_obs)
tmp[adata.obs['predicted_doublets'].values==True]=True
adata.obs['predicted_doublets']=tmp
[19]:
print(f"# of cells with scrublet score >= 0.2: {np.sum(adata.obs['scrublet_score']>=0.2)} \n# of predicted doublets: {np.sum(adata.obs['predicted_doublets'])}")
# of cells with scrublet score >= 0.2: 1510
# of predicted doublets: 373
Normalization#
[20]:
sc.pp.normalize_total(adata, target_sum=1e4)
sc.pp.log1p(adata)
adata.layers['log1p']=adata.X.copy()
normalizing counts per cell
finished (0:00:00)
INFOG normalization#
We use PIASO’s infog to normalize the data and identify a highly variable set of genes.
[21]:
%%time
piaso.tl.infog(adata,
layer='raw',
n_top_genes=3000,)
The normalized data is saved as `infog` in `adata.layers`.
The highly variable genes are saved as `highly_variable` in `adata.var`.
Finished INFOG normalization.
CPU times: user 19 s, sys: 8.06 s, total: 27.1 s
Wall time: 27.1 s
SVD dimensionality reduction and visualization#
[22]:
piaso.tl.runSVD(adata,
use_highly_variable=True,
n_components=50,
random_state=10,
key_added='X_svd',
layer='infog')
[23]:
%%time
sc.pp.neighbors(adata,
use_rep='X_svd',
n_neighbors=15,
random_state=10,
knn=True,
method="umap")
sc.tl.umap(adata)
computing neighbors
finished: added to `.uns['neighbors']`
`.obsp['distances']`, distances for each pair of neighbors
`.obsp['connectivities']`, weighted adjacency matrix (0:00:40)
computing UMAP
finished: added
'X_umap', UMAP coordinates (adata.obsm)
'umap', UMAP parameters (adata.uns) (0:00:36)
CPU times: user 1min 30s, sys: 685 ms, total: 1min 30s
Wall time: 1min 16s
[24]:
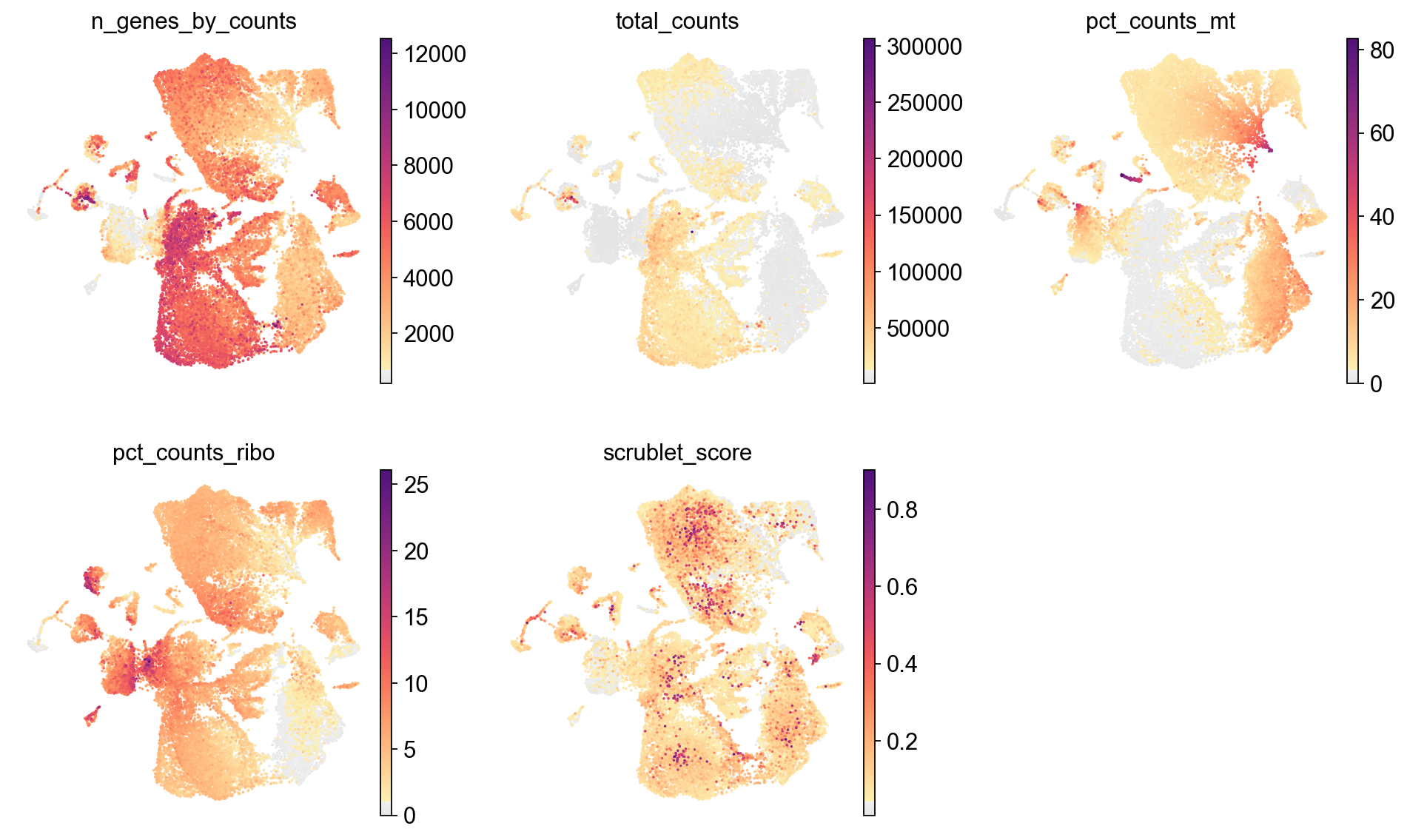
sc.pl.umap(adata,
color=['n_genes_by_counts', 'total_counts','pct_counts_mt','pct_counts_ribo', 'scrublet_score'],
cmap=piaso.pl.color.c_color1,
palette=piaso.pl.color.d_color1,
ncols=3,
size=10,
frameon=False)
[26]:
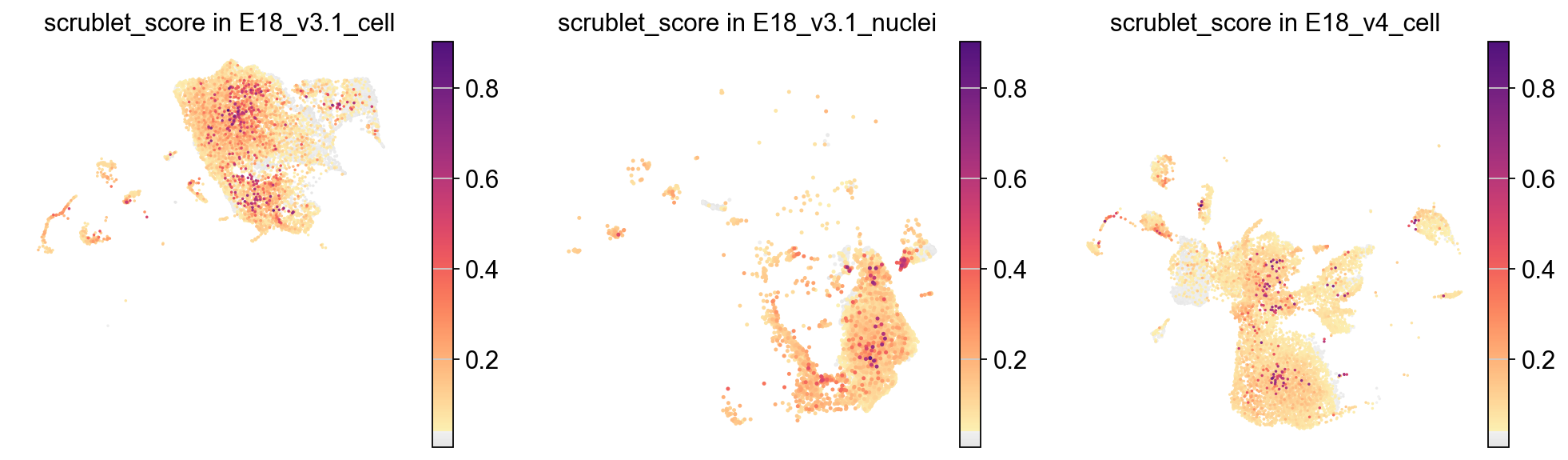
piaso.pl.plot_embeddings_split(adata,
color='scrublet_score',
cmap=piaso.pl.color.c_color1,
splitby='Sample',
ncol=3,
frameon=False)
Leiden clustering#
[27]:
%%time
sc.tl.leiden(adata,resolution=0.5,key_added='Leiden')
running Leiden clustering
finished: found 24 clusters and added
'Leiden', the cluster labels (adata.obs, categorical) (0:00:03)
CPU times: user 3.85 s, sys: 114 ms, total: 3.96 s
Wall time: 3.96 s
[28]:
sc.pl.umap(adata,
color=['Leiden'],
palette=piaso.pl.color.d_color1,
legend_fontsize=12,
legend_fontoutline=2,
legend_loc='on data',
ncols=1,
size=10,
frameon=False)
WARNING: Length of palette colors is smaller than the number of categories (palette length: 19, categories length: 24. Some categories will have the same color.
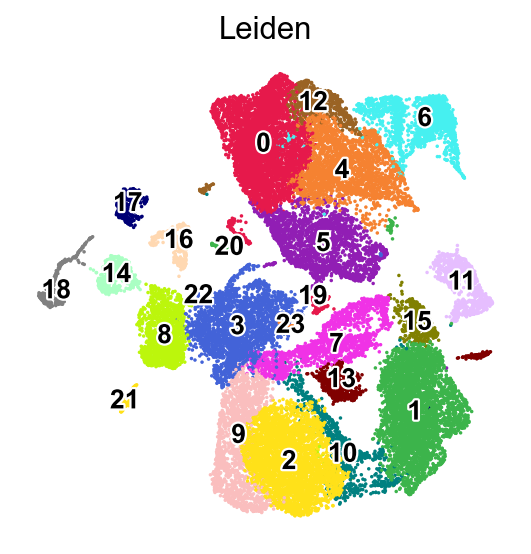
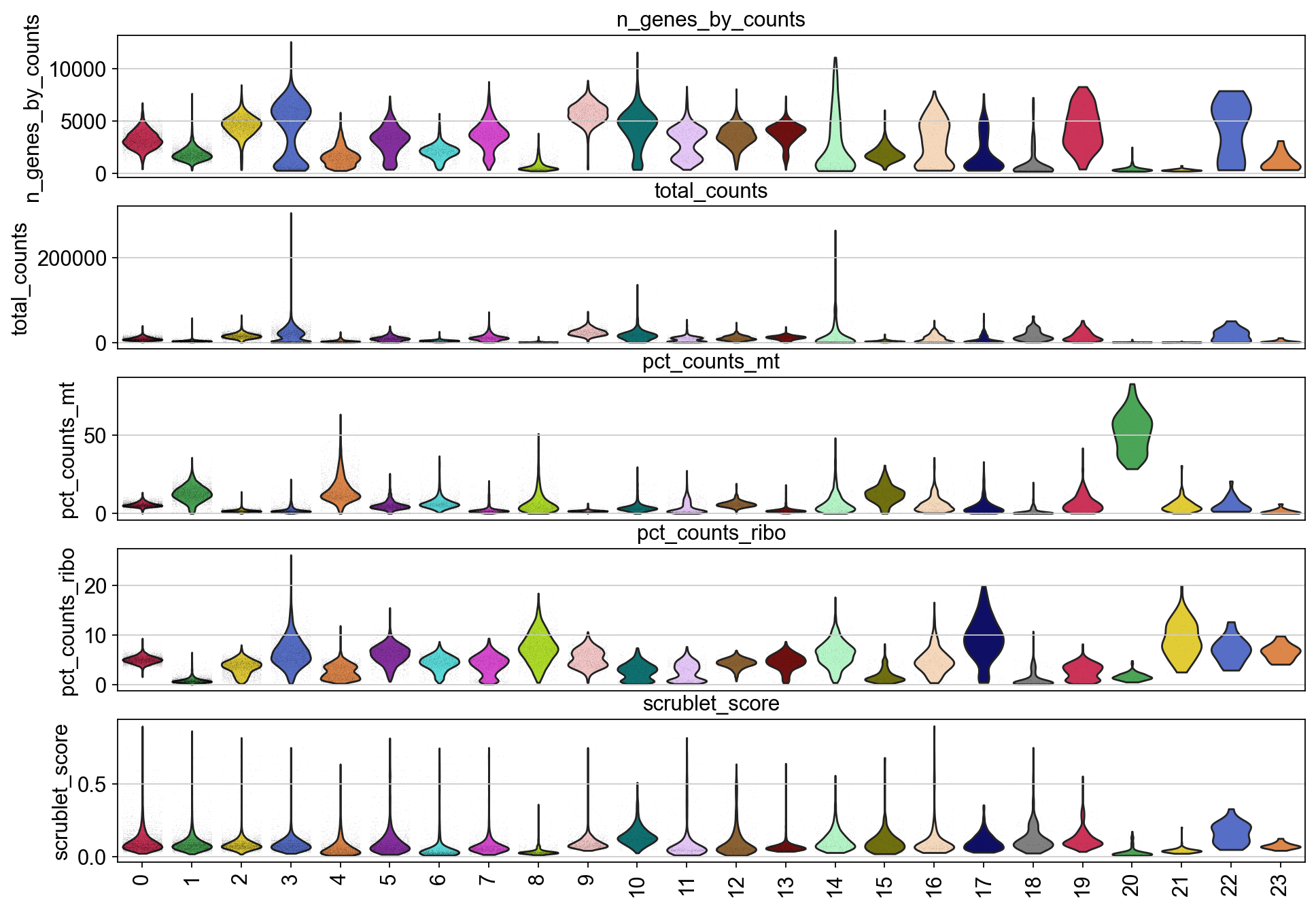
[29]:
piaso.pl.plot_features_violin(adata,
['n_genes_by_counts', 'total_counts', 'pct_counts_mt','pct_counts_ribo', 'scrublet_score'],
groupby='Leiden')
Identify marker genes with COSG#
[30]:
%%time
n_gene=30
cosg.cosg(adata,
key_added='cosg',
use_raw=False,
layer='log1p',
mu=100,
expressed_pct=0.1,
remove_lowly_expressed=True,
n_genes_user=100,
groupby='Leiden')
CPU times: user 6.57 s, sys: 2.75 s, total: 9.32 s
Wall time: 9.32 s
We can use a dendrogram dot plot to visualize the expression of the top three marker genes of each Leiden cluster.
[31]:
sc.tl.dendrogram(adata,groupby='Leiden',use_rep='X_svd')
df_tmp=pd.DataFrame(adata.uns['cosg']['names'][:3,]).T
df_tmp=df_tmp.reindex(adata.uns['dendrogram_'+'Leiden']['categories_ordered'])
marker_genes_list={idx: list(row.values) for idx, row in df_tmp.iterrows()}
marker_genes_list = {k: v for k, v in marker_genes_list.items() if not any(isinstance(x, float) for x in v)}
sc.pl.dotplot(adata,
marker_genes_list,
groupby='Leiden',
layer='log1p',
dendrogram=True,
swap_axes=False,
standard_scale='var',
cmap='Spectral_r')
Storing dendrogram info using `.uns['dendrogram_Leiden']`
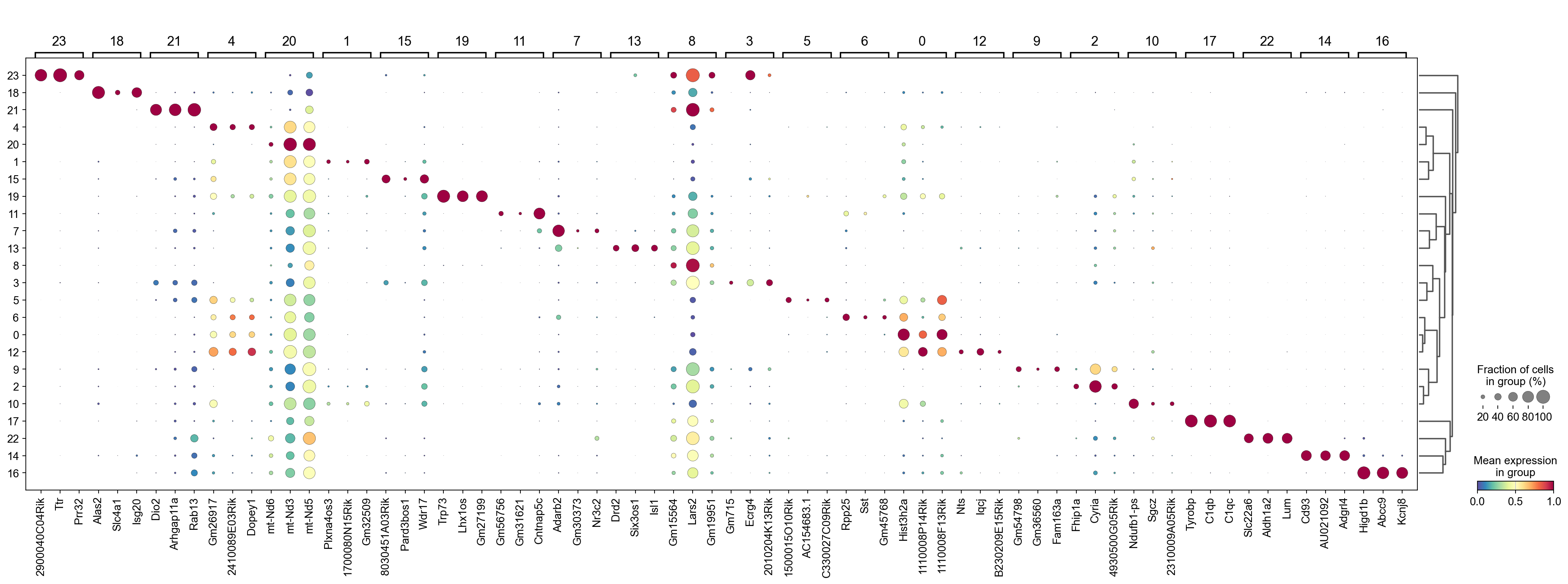
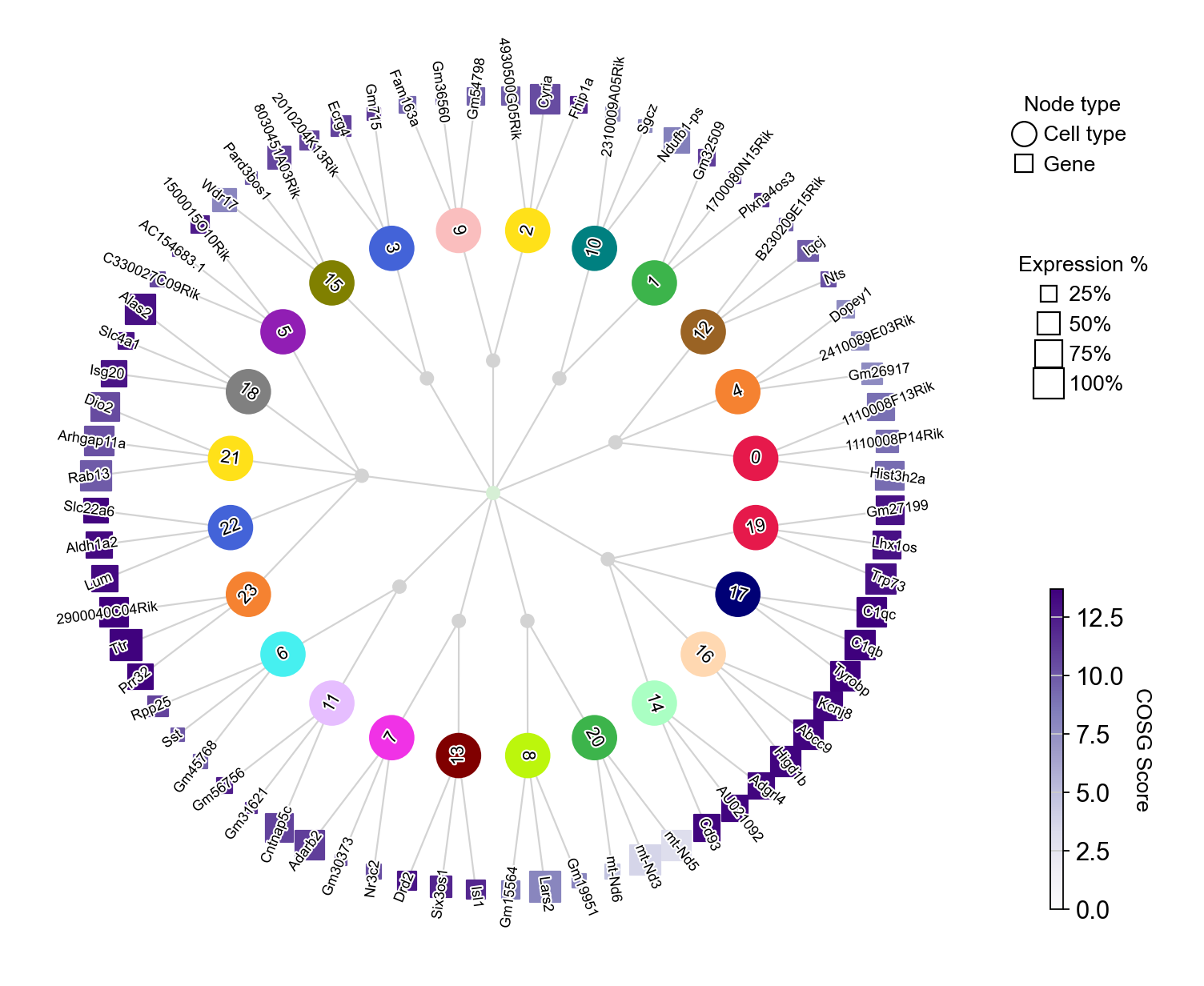
Another way to visualize the expression of the top three marker genes from each Leiden cluster is by using COSG’s plotMarkerDendrogram method to create a circular dendrogram.
[32]:
cosg.plotMarkerDendrogram(
adata,
group_by="Leiden",
use_rep="X_svd",
calculate_dendrogram_on_cosg_scores=True,
top_n_genes=3,
radius_step=4.5,
cmap="Purples",
gene_label_offset=0.25,
gene_label_color="black",
linkage_method="ward",
distance_metric="correlation",
hierarchy_merge_scale=0,
collapse_scale=0.5,
add_cluster_node_for_single_node_cluster=True,
palette=None,
figure_size= (10, 10),
colorbar_width=0.01,
gene_color_min=0,
gene_color_max=None,
show_figure=True,
)
Marker genes of an individual cluster#
We can use dotplots and UMAPs to visualize the expression of the top marker genes in a selected cluster, which can be used to evaluate cluster quality.
[33]:
marker_gene=pd.DataFrame(adata.uns['cosg']['names'])
[34]:
cluster_check='11'
marker_gene[cluster_check].values
[34]:
array(['Gm56756', 'Gm31621', 'Cntnap5c', 'Dlgap2', 'Gm13986', 'Dpp10',
'Nxph1', '4930555F03Rik', 'Gm2516', 'Gm45323', 'Epb41l4a', 'Chrm3',
'Gm45341', 'Syndig1', 'Cntnap3', 'Kcnmb2', 'Ackr3', 'Sntg2', 'Mkx',
'Cacng3', 'Nxph2', 'Gria4', 'Rbms3', 'Gria1', 'Nell1', 'Erbb4',
'Tox2', 'Ror2', 'Greb1l', 'Neb', 'Neto1', 'Vat1l', 'C130073E24Rik',
'Ptprt', 'Cntnap4', 'Kcns3', 'Amph', 'Maf', 'Dgkg', 'Gm47664',
'Dpp6', 'Adamtsl1', 'Iqsec1', 'Fam135b', 'Dlx1as', 'Kcnc2', 'Lhx6',
'Grm5', 'Gm38505', 'Dlgap1', 'Tafa5', 'Gabrg3', 'Rph3a', 'Pde4dip',
'Edaradd', 'Gm20754', 'Fsip1', '4930415C11Rik', '4930509J09Rik',
'Efcab6', 'Fgd3', 'Spats2l', 'Cdk14', 'Ptchd4', 'Ryr2', 'Gm14204',
'Lhfpl3', 'Csrnp3', 'Sox1ot', 'Elfn1', 'Shisa6', 'Ripor2', 'Nrxn3',
'Syt1', 'Rps6ka5', 'Grik1', 'Sox2ot', 'Gm26691', 'Xkr4', 'Tgfb3',
'A330076H08Rik', 'Brinp3', 'Cbfa2t3', 'Fgf12', 'Bend4', 'Cdr1os',
'Elmo1', 'Npy', 'Zfp536', 'Prickle2', 'Slc6a1', 'Cacnb4', 'Grip1',
'Pde2a', 'Gm19938', 'Gad1os', 'Tbc1d9', 'Galnt13', 'Brinp1',
'Kcnip1'], dtype=object)
The dot plot below displays the expression of the top marker genes from cluster 8 across all clusters. For cluster 8 to be considered high quality, its top marker genes should be primarily specific to cluster 8, showing little to no expression in other clusters.
[35]:
sc.pl.dotplot(adata,
marker_gene[cluster_check].values[:50],
groupby='Leiden',
dendrogram=False,
swap_axes=True,
standard_scale='var',
cmap='Spectral_r')
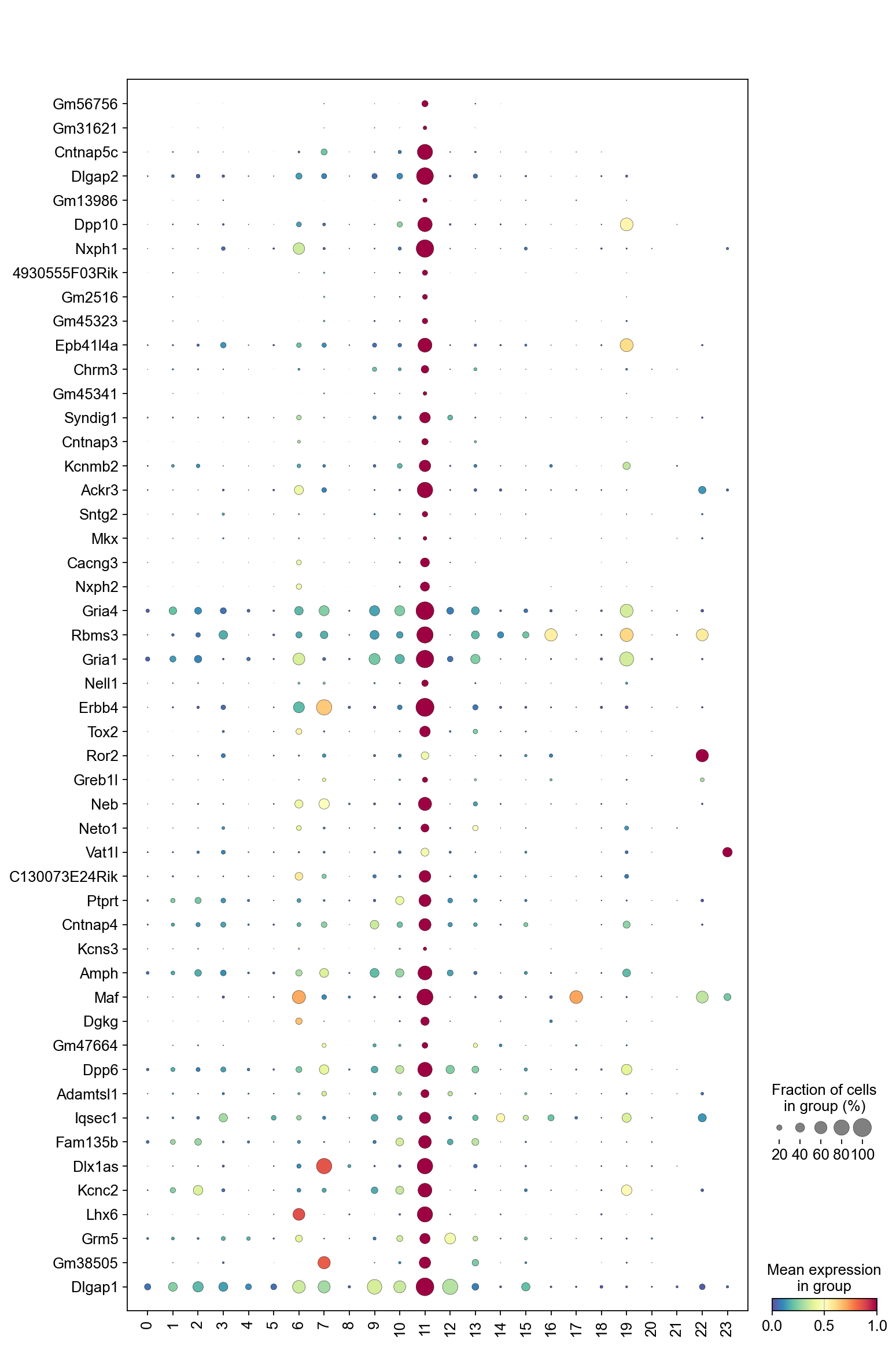
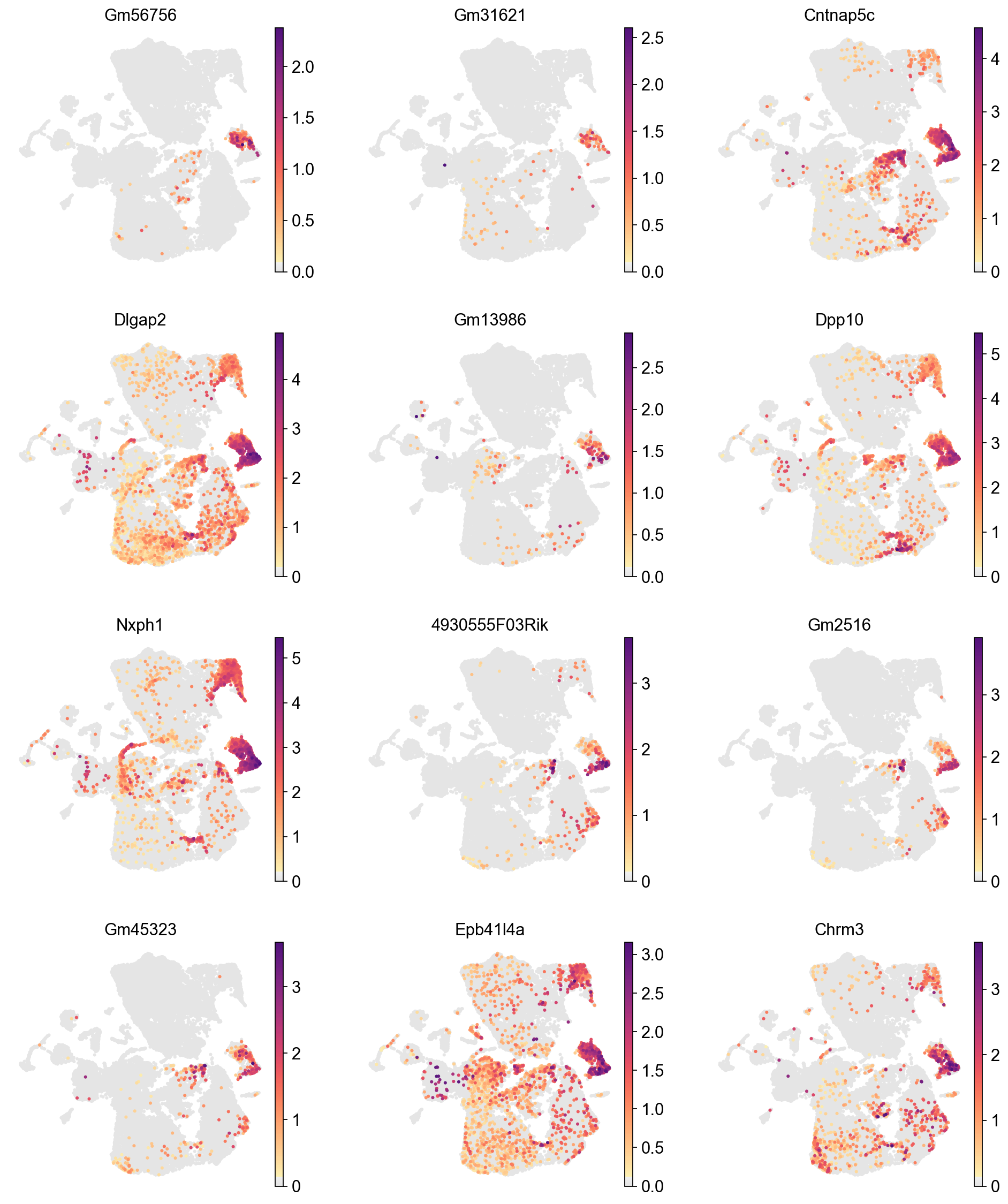
We can visualize the expression of the top 12 genes from cluster 8 on UMAPs to assess whether their expression is specific to the cluster 8 region. This involves comparing the location of cluster 8 on the Leiden cluster UMAP with the gene expression UMAPs. In a high-quality cluster, marker gene expression should be predominantly localized to the cluster 8 area.
[36]:
sc.pl.umap(adata,
color=marker_gene[cluster_check][:12],
palette=piaso.pl.color.d_color1,
cmap=piaso.pl.color.c_color1,
layer='log1p',
legend_fontsize=12,
legend_fontoutline=2,
legend_loc='on data',
ncols=3,
size=30,
frameon=False)
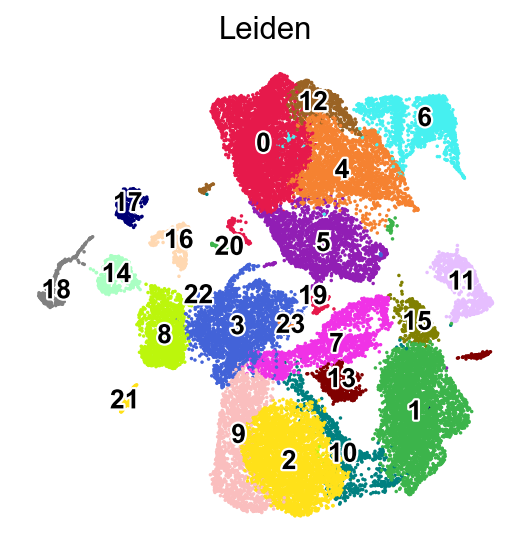
[37]:
sc.pl.umap(adata,
color=['Leiden'],
palette=piaso.pl.color.d_color1,
cmap=piaso.pl.color.c_color1,
legend_fontsize=12,
legend_fontoutline=2,
legend_loc='on data',
ncols=1,
size=10,
frameon=False)
WARNING: Length of palette colors is smaller than the number of categories (palette length: 19, categories length: 24. Some categories will have the same color.
This pipeline is still in development. The next steps include using a reference dataset to annotate cell types with PIASO’s predictCellTypesByGDR, followed by multiple iterations of low-quality cluster removal and re-clustering until well-defined clusters are obtained.
